Why Investigator Meetings are Important for the Success of a Clinical Study
While there continues to be tremendous innovation throughout the clinical trial industry, everything from decentralized trials to remote monitoring to eCOA solutions and everything in between, having motivated and highly-trained healthcare professionals (HCPs) remains paramount to achieving quality patient enrollment and clinical study data.
Despite a shift to virtual meetings during the pandemic, pharma/biotech sponsor companies continue to prioritize relationship-building with their sites in person. There has been a significant return to face-to-face and hybrid meetings over the last few years, with a current preference for full face-to-face meeting format. By collaborating in person, HCPs are better able to share opinions, discuss ideas, learn collaboratively, and build stronger relationships. For sponsors, there is a need to focus on current market trends to enhance the engagement and quality of investigator meetings to achieve successful outcomes.
The Challenges of Planning Investigator Meetings
The clinical research space is facing a significant challenge in limited resources, which puts a strain on the ecosystem and individuals. This is felt across the board at the site level, as well as pharma, biotech, and CROs. This challenge is further magnified within investigator meeting planning. Miller Tanner Associates (MTA) often sees companies initiate planning much too late in their overall study timeline and this unfortunately has a critical downstream impact on the success of programs.
This lack of resources and shorter planning time directly impacts attendance ratios, costs, and the overall success of the investigator meeting. For example, if HCPs are not receiving enough advanced notification with a save-the-date or an invitation, then the per-attendee travel costs increase and, ultimately, attendance is significantly impacted. We recently had an expedited meeting with international travel in which airfare costs had tripled per ticket compared to prices available two to three weeks prior had the event been planned further in advance. Some of this is also driven by the market demand challenges with venue availability and staffing for third-party vendors such as ground transportation and hotel staff.
By implementing a few proactive strategies, sponsors can mitigate potential challenges to ensure a more successful investigator meeting.
Lead Time Leads to Success
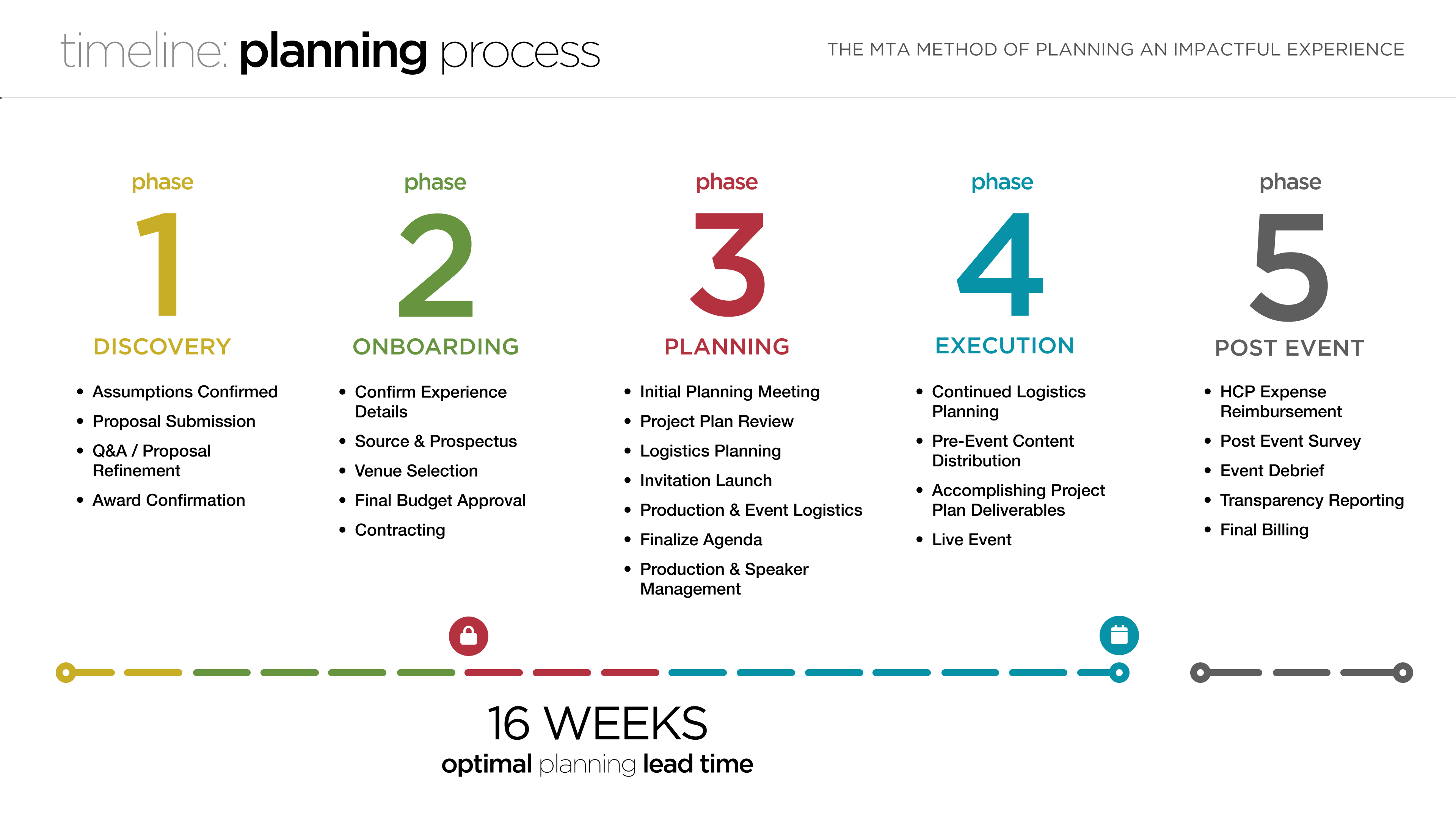
One key factor for guaranteeing a successful investigator meeting is lead time. A 16-week planning lead time for an investigator meeting will significantly improve the success of the meeting. An adequate planning timeline allows the study team to clearly define the agenda to achieve their goals and objectives, work through venue sourcing/selection/contracting, launch invitations to provide adequate notification to the HCPs, and execute all other detailed logistics planning including attendee travel bookings, content finalization, etc.
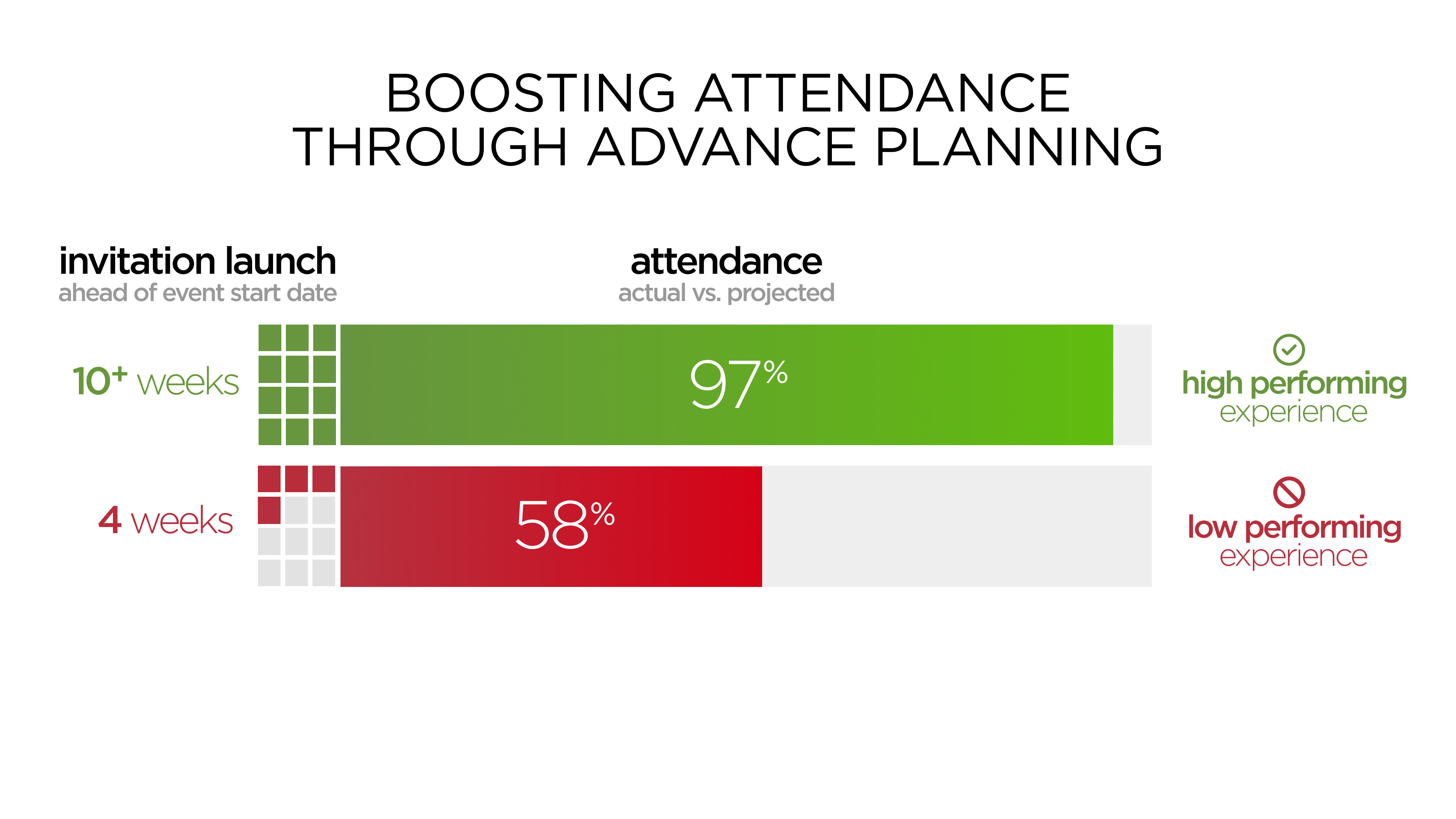
Miller Tanner Associates’ research has shown a direct correlation and significant increase in investigator meeting attendance as a result of more advanced lead times. For example, a recent high-performing event with a 90-day advance launch of invitation ahead of an investigator meeting resulted in 97% attendance ratio (actual attendees as a percentage of projected). Comparatively, a low-performing event with 31 days from invitation launch to event start date resulted in only 58% attendance. The extra 60-day lead time on invitation launch resulted in a significant increase in attendance ratio leading to the large majority of sites receiving consistent, high-quality training.
We also conducted an in-depth analysis of life science events throughout 2023 and looked at reasons for decline in attendance. The data indicated that 85% of declines were due to the timing/lead time of the event for HCPs. Lead time is truly the greatest opportunity for improving conversion rate and increasing attendance ratio.
Driving Audience Engagement
Longer lead time also allows for a more focused, intentional agenda that can be structured to accommodate various learning styles and designed strategically, whether that be with more targeted presentations, collaborative breakouts, and networking opportunities for peer engagement. Content engagement can be strengthened by incorporating polling, Q&A sessions, and gamification into the agenda. The agenda can be optimized to not only address the meeting goals, but also engage the audience for overall maximum learning outcomes.
To increase audience engagement, consider a more creative approach. For more complex training material such as complicated lab sample collections or the use of a unique device, have the vendor do a hands-on training during breaks. Again, incorporating polling and gamification into key sessions that are critical for learning outcomes result in higher learning outcome achievement. At MTA, we’ve implemented a variety of creative strategies at investigator meetings to great effect. One investigator meeting utilized recording Google glasses to instruct and observe remote sonographer trainees, while another featured interactive learning through headphone-equipped exhibit halls. With unlimited ways to engage attendees, the possibilities are endless – it all depends on your goals for the meeting.
Production is Worth the Investment
Production is critical for a successful investigator meeting. Having intentionality behind your event’s production leads to the best return on your investment. To ensure that the message is conveyed effectively to the audience, a professional production team should be in charge of executing the audio-visual elements, coaching speakers through technical rehearsals and making certain that the event’s agenda runs seamlessly. Additionally, sharing pre-meeting or post-meeting content with those unable to attend, managing simultaneous interpretation, executing a hybrid format, or recording the meeting for future trainings are other reasons to consider investing in an expert production team.
Additionally, hybrid meetings are becoming increasingly popular, and it takes a lot of effort to make the experience seamless. The key is to create a shared experience by leveraging technology to make both the virtual and face-to-face audiences feel like they are part of the same event. Designing a shared experience for both audiences, such as polling and surveying with results shown in real-time for both audiences, can lead to a more interactive and engaging hybrid experience.
Complying with Compliance
Changes to compliance guidelines are frequent and complex. Several recent updates within European countries such as Belgium and France, have made it even more challenging to meet certain requirements for hotel, meal, and travel caps for HCP meeting attendees. More advanced planning and longer lead times are required to ensure that compliance guidelines are followed and timelines are achieved with the appropriate regulatory approvals.
Best Practice for Best Results
Most of the challenges that sponsors face when planning an investigator meeting can be overcome by simply implementing a minimum 16-week planning lead time. By planning earlier, sponsors can better regulate costs, significantly enhance attendance ratios, determine production needs with precision, and allow ample time to integrate an intentional strategy into the agenda and fully develop content with audience engagement in mind. This will result in a more engaging experience for your attendees, leading to better training outcomes and a more successful clinical study.
This article was originally published in the Outsourcing Clinical Trials Handbook.